RHEACELL, a German biotech company specialized in the development of stem cell therapies, and AOP Health, a Europe-based leader in integrated therapies for rare diseases and in intensive care, are forming a strategic alliance. This partnership marks a crucial step toward making breakthrough therapies available to patients with high unmet medical needs – particularly those affected by Epidermolysis bullosa (EB or “butterfly children’s” disease) and chronic venous ulcers. Both conditions currently have limited treatment options, severely impacting the lives of patients and their families.
Two novel therapies from RHEACELL’s pipeline are currently in Phase 3 clinical development. Combining RHEACELL’s cutting-edge R&D capabilities with AOP Health’s proven expertise in bringing rare disease therapies to patients in Europe, this partnership underscores both companies’ strong commitment to addressing high unmet medical needs.
With 30 years of experience and a growing presence in more than 50 countries, AOP Health complements this ambition by providing the expertise needed to make RHEACELL’s cell therapies available not only in Europe, but also in the Middle East, North Africa, Türkiye, and Israel.
Promising cell therapy
Cell-based regenerative therapies are gaining increasing importance, particularly for diseases with currently limited therapeutic options. For over 20 years, RHEACELL has focused on the research and development of so-called stromal cells – stabilizing cells obtained from donor skin. RHEACELL’s cell therapy is only the second stromal cell product to receive national marketing authorization from the Paul Ehrlich Institute in Germany for the treatment of therapy-resistant, chronic venous ulcers (“leg ulcers”) on the basis of positive phase 2 clinical trial data.[1],[2]
 An innovative mechanism of action
An innovative mechanism of action
RHEACELL’s cell therapy is based on a specific type of cells, so-called ABCB5+ mesenchymal stromal cells, with anti-inflammatory and immunomodulatory properties. These cells represent a promising new therapeutic approach for EB and chronic venous ulcers. In patients with EB, this therapy is administered systemically and can thus stimulate both internal and external wound healing by restoring the normal physiological function of affected tissues – an innovative mechanism of action. This can lead to a reduction in the number of existing wounds and help prevent the formation of new ones.[3]
Building on positive Phase 2 results and positive feedback from regulatory authorities, two Phase 3 clinical trials are currently underway, and a first EMA submission is already expected in 2026.
About Epidermolysis bullosa (EB)
EB is a congenital and currently incurable condition that can affect not only the skin but also internal organs. With approximately 500,000 people affected worldwide[4], EB is a rare disease that severely impacts patients’ quality of life. In this genetic disorder, the skin is as fragile as a butterfly’s wing. Even minor mechanical stress or friction can cause blistering and painful chronic wounds.
The patient organization DEBRA describes EB as “the worst disease you’ve never heard of.” DEBRA was founded in 1995 as a patient organization by patients, families, and physicians with the goal of facilitating exchange and providing support to people living with EB. One of DEBRA Austria’s key initiatives is the EB-Haus Austria, located at the Salzburg University Hospital campus. It serves as a center of excellence for Epidermolysis bullosa and is also the world’s first specialized clinic for “butterfly children”.
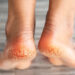
About Chronic Venous Ulcers (CVU)
Venous ulcers are chronic sores that result from prolonged venous insufficiency, primarily affecting older adults. These ulcers develop due to poor blood flow in the veins, leading to tissue breakdown, especially in the lower extremities. They are the most common type of leg ulcers, accounting for 60–80% of cases.
[1] https://www.rheacell.com/pdf/Gebrauchs-und-Fachinformation_AMESANAR.pdf
[2] https://www.pei.de/SiteGlobals/Forms/Suche/Arzneimittelsuche_Formular.html
[3] Kiritsi et al. Clinical trial of ABCB5-positive mesenchymal stem cells for recessive dystrophic epidermolysis bullosa. JCI Insight 2021;6:e151922
[4] Rashidghamat, E. et al (2017). Persistent and rare diseases research. 2017; 6(1):6-20. DOI: 10.5582/irdr.2017.0100